Gold is one of them most sought after materials in the world - with intrinsic value it's no match for fiat currency; it's not only a stable investment but also a necessity for technology. But what's the back story on gold? What is it made up of and what is so good about it? Below we detail the properties of gold including the atomic radius and boiling point.
Gold is ductile
Gold is ductile, meaning it can be thinned out and stretched to the greatest of lengths: no, seriously. One measly ounce of gold can stretch as far as 50 miles! Why? Because of the FCC lattice crystal structures it is made up of.
Even with its impressive ductility, platinum beats out gold for the most ductile metal. But gold wins for most malleable. This is shown in gold flakes, which are essentially extremely thin sheets of gold. It is so malleable that it can be beaten thin enough to become transparent.
Electricity conductor
The collection of delocalized electrons surrounding gold atoms is what creates a charge, which is what gives it its ability to conduct electricity. The electrons work together to move from positive to negative, which in turn conducts electricity and allows for our technological devices to work.
Gold is malleable
As mentioned above, gold is the most malleable metal in the world. It is so easily flattened into leafs and stretched into thin wires which makes it a great use for both technology and art. Gold leafing is an ancient art form which uses extremely thing leafs of gold to create a masterpiece. When it comes to wiring, gold is stretched into the thinnest of wires and fit into our technological devices - this is what makes it so sought after in the technology field. Because of this, gold has real world uses, making it a great asset to invest in.
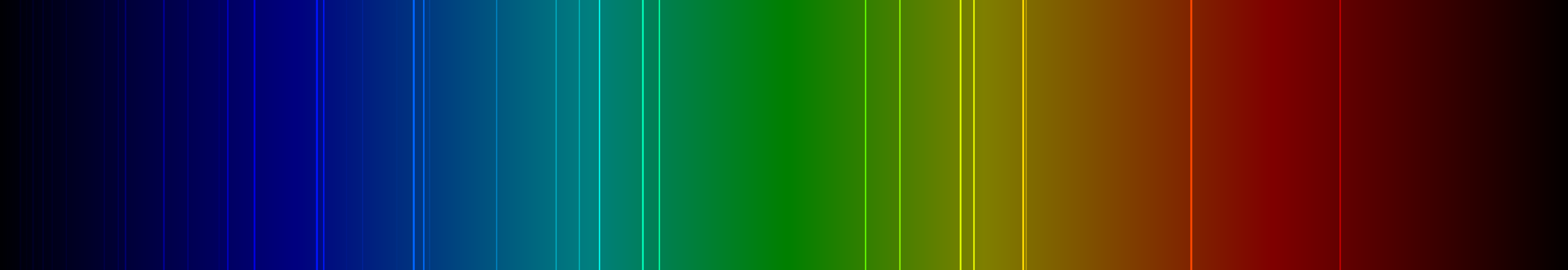
Gold is extremely reflective
Gold has almost 100% reflectivity over 700 nm which reduces around 500 nm. The longer the wavelength, the better gold's reflectivity is because of the electrons' ability to move from one side to another, to then reflect the radiation or light.
Infrared
Gold reflects just about 99% of the infrared light it comes in contact with. This is yet another reason it works so well in technology; it keeps other material cool and does not have any chance of overheating. Gold is best at reflecting red and yellow light, which is at the lower end of the spectrum.
